Overview
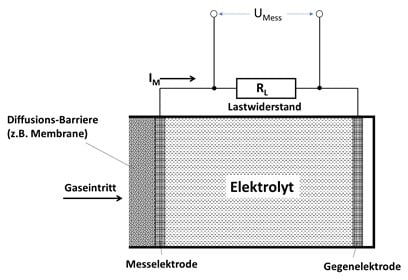
The O2.sens from Wi.Tec is based on an electrochemical process. In this process, a chemical reaction takes place with the oxygen to be measured and a liquid electrolyte. In this reaction, 4 electrons are released per oxygen molecule. The resulting sensor current IM or the measurement voltage UM increases proportionally with the number of reacting O2 molecules. This results in a sensor characteristic that increases linearly with increasing O2 concentration.
Basic structure
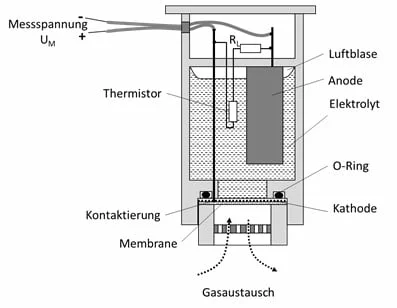
The liquid electrolyte is located in a closed chamber, which is sealed on the gas side with a permeable membrane. The oxygen reaches the electrolyte through this membrane and then leads to the measuring effect described. The anode material is consumed during this reaction, so that the service life of this sensor is limited. A membrane with a high permeability leads to a high and rapid signal change (= short response time), but also to a shorter service life, since more chemical reactions take place over time. Membranes with a low permeability therefore show the opposite behavior.